Cell Systems and Drug Safety
Selectivity
Target selectivity is an important aspect of any drug molecule, and certainly a parameter to be optimized. That is not trivial for a number of reasons. First of all hundreds of drug targets (receptors, enzymes, ion channels) exist, and no single lab in the world has assays for all of them.
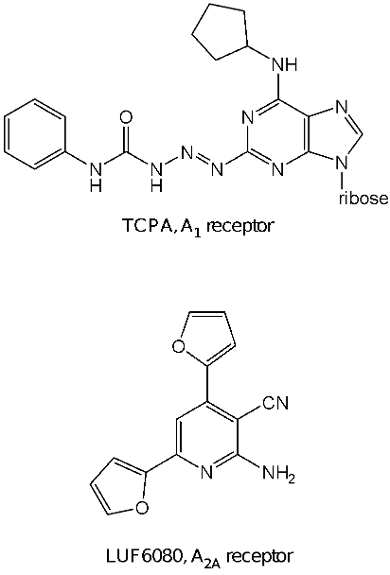
So, routinely, ligands tend to be tested against a small number of related targets, such as the four subtypes of adenosine receptors. Secondly, selectivity often is species-dependent. Particularly, compounds from the past, developed in animal studies, may have good selectivity in e.g. rodent tissue, but fail to demonstrate such selectivity in human tissues. Vice versa, compounds profiled in humanized assays, e.g. CHO cells overexpressing a human receptor, may face difficulties in further development in advanced animal models of disease. As an example we have focused on the synthesis of truly selective ligands for human adenosine receptors, and identified TCPA as a highly selective agonist for the human adenosine A1 receptor and LUF6080 with reasonable selectivity as an antagonist for the adenosine A2A receptor.
We are now very interested in the concept of ‘kinetic selectivity’. Compounds may have different kinetic profiles of interaction at related drug targets. Tiotropium is a nice example – it has almost equal affinity for all five muscarinic receptor subtypes, but it sits on the M3 receptor for a very long time, unlike the other four. In practice, this means that tiotropium is quite selective in its actions in vivo, being used in the treatment of COPD. We have recently explored this phenomenon ourselves for the adenosine and tachykinin (NK1) receptors.
Last but not least, we pay significant attention to the computational prediction of selectivity. This is by no means trivial, but the first steps have been set. We’re helped here by the high-resolution crystal structures of e.g. adenosine receptors, a research area in which we are actively involved.
Related publications
- Martella A., Sijben H., Rufer A.C., Grether U., Fingerle J., Ullmer C., Hartung T., IJzerman A.P., van der Stelt M., Heitman L.H., A Novel Selective Inverse Agonist of the CB(2) Receptor as a Radiolabeled Tool Compound for Kinetic Binding Studies. Mol Pharmacol 2017, 92(4): 389-400.
- Alachouzos G., Lenselink E.B., Mulder-Krieger T., de Vries H., IJzerman A.P., Louvel J., Synthesis and evaluation of N-substituted 2-amino-4,5-diarylpyrimidines as selective adenosine A1 receptor antagonists. Eur J Med Chem 2017, 125: 586-602.
- Guo D., Dijksteel G.S., van Duijl T., Heezen M., Heitman L.H., IJzerman A.P., Equilibrium and kinetic selectivity profiling on the human adenosine receptors. Biochem Pharmacol. 2016 Apr 1;105: 34-41.
- Mantri M., de Graaf O., van Veldhoven J., Göblyös A., Von Frijtag Drabbe Künzel J.K., Mulder-Krieger T., Link R., de Vries H., Beukers M.W., Brussee J., IJzerman A.P., 2-Amino-6-furan-2-yl-4-substituted nicotinonitriles as A2A adenosine receptor antagonists. J Med Chem 2008, 51(15): 4449-55.
- Beukers M.W., Wanner M.J., Von Frijtag Drabbe Künzel J.K., Klaasse E.C., IJzerman A.P., Koomen G.J., N6-cyclopentyl-2-(3-phenylaminocarbonyltriazene-1-yl)adenosine (TCPA), a very selective agonist with high affinity for the human adenosine A1 receptor. J Med Chem 2003, 46(8): 1492-503.

