Research project
Mathematical modelling of adverse outcome pathways
In this project, we aim to develop mathematical models to mechanistically and quantitatively predict the dynamics of cellular stress pathway activation and its relation with toxic effects when cells are exposed to various toxicants.
- Contact
- Joost Beltman
- Funding
- NWO grant Meer Kennis met Minder Dieren (MKMD)
- Horizon 2020 EU grant EUTOXRISK
- IMI2 grant eTransafe
There is a strong need to develop novel toxicological approaches that incorporate knowledge on the mechanisms by which toxicants cause adverse effects at cell and tissue level. Computational approaches like mathematical modelling of pathway dynamics can contribute to a better mechanistic understanding and prediction of stress pathway activation and their relation to adverse effects.
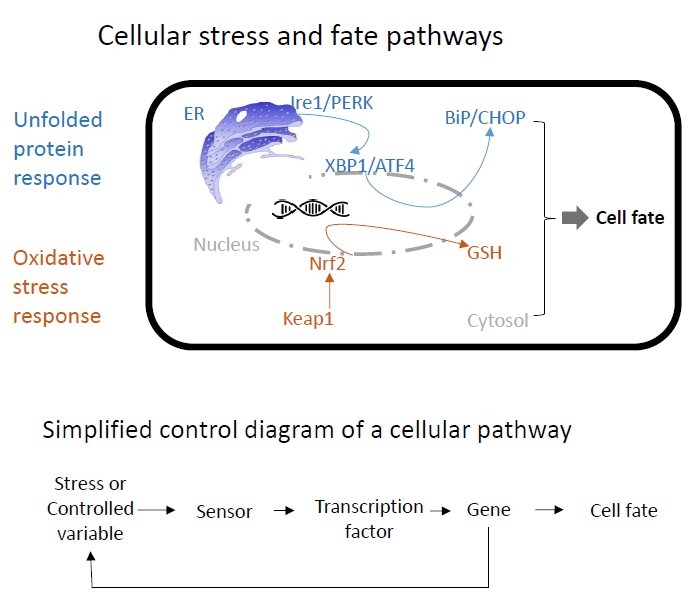
Material and Methods. We exploit in-house imaging data sets in which liver cells that express fluorescent reporters for relevant proteins within cellular stress pathways (e.g., the oxidative stress response and the unfolded protein response) are exposed to toxicants (see figure). These data are incorporated into ordinary differential equation models that describe stress pathway dynamics and the fate of single cells such as their death. Thus, we aim for a highly interdisciplinary approach in which models are fitted to the dynamic experimental data in order to make predictions of adverse effects.
LACDR and Leiden University. This project fits the ambition of the LACDR to apply computational approaches in drug research, in this case specifically applied to the prediction of drug safety. The presence of state-of-the-art imaging equipment at the Cell Observatory enables access to the required data for these projects.
Societal relevance. Drug safety is an important topic during the development of novel drugs. For example, drug-induced liver injury (DILI), a frequent cause of acute liver failure, is a significant threat to human health and is very difficult to predict. As a consequence, new drugs are often found to be causing DILI at a very late stage of drug development, making this a very costly procedure. Therefore, we urgently need novel approaches for the prediction of adverse effects. We aim to contribute to such predictions by our interdisciplinary approach involving in vitro dynamical imaging and mathematical modelling.
