Single-layer graphene proton conducting membrane in electrochemical devices
- Date
- Friday 25 October 2024
- Time
- Address
-
Gorlaeus Building
Einsteinweg 55
2333 CC Leiden - Room
- BM.1.23
Weizhe Zhanga,b, Arnaud Thevenon a, Pieter Bruijnincx a, Grégory F. Schneider b
a Organic Chemistry and Catalysis, Institute for Sustainable and Circular Chemistry, Faculty of Science, Utrecht University, Universiteitsweg 99, Utrecht, The Netherlands.
b Leiden Institute of Chemistry, Faculty of Science, Leiden University, Einsteinweg 55, 2333CC Leiden, The Netherlands.
Abstract
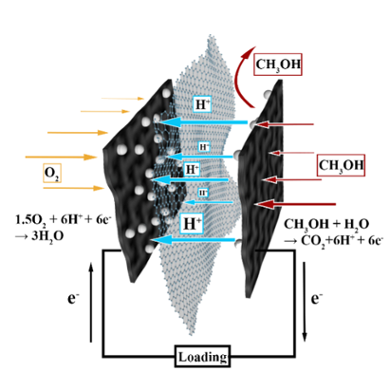
Advances in electrochemical reactors offer innovative strategies to address energy and chemical conversion demands, targeting clean and sustainable solutions. A critical component in these devices is the membrane, which separates half-reactions, regulates mass transport, and ultimately determines device performance. An optimized proton membrane must satisfy two primary criteria: proton permeability and selectivity. Proton permeability is essential for achieving high electrical efficiency, while selectivity prevents reactants crossover between electrodes, which can significantly degrade catalyst performance[1]. Pristine graphene inherently meets these criteria due to its impermeable basal plane and inherent proton conductivity, influenced by nanoscale ripples, corrugations, particularly in monolayer graphene oxide, and hydrogenated graphene[2]. Here, we first review recent advances in electrochemical applications, including plastic recycling, CO2 reduction, and power generation, with a particular focus on the role of membranes in device performance. We then present our recent work on enhancing the proton conductance of graphene without compromising selectivity. This was achieved by chemically functionalizing monolayer graphene to introduce sulfophenylated sp3 dislocations. The resulting centimeter-scale, single-layer graphene was tested in direct methanol fuel cells, demonstrating superior performance in both proton conductance and selectivity over methanol. By creating proton-conductive and selective pathways through graphene, we reveal a strategy to rationalize transmembrane proton transport through 2D materials, offering a significant advancement in the development of high-performance electrochemical devices.
Acknowledgements
WZ thanks the Dutch Research Council (NWO) for funding via OCENW.XS21.4.035. G.F. S thanks (FP/2007-2013)/ERC Grant Agreement no. 335879 project acronym “Biographene” and the Netherlands Organization for Scientific Research (NWO) VIDI 723.013.007.
References
- Park, H. B. et al. Science 2017, 356, eaab0530.
- Tong, J. et al. Nature 2024, 630, 619-624.
